Ideal Tips About How To Get A Clia Number

Email the international laboratory clia certification.
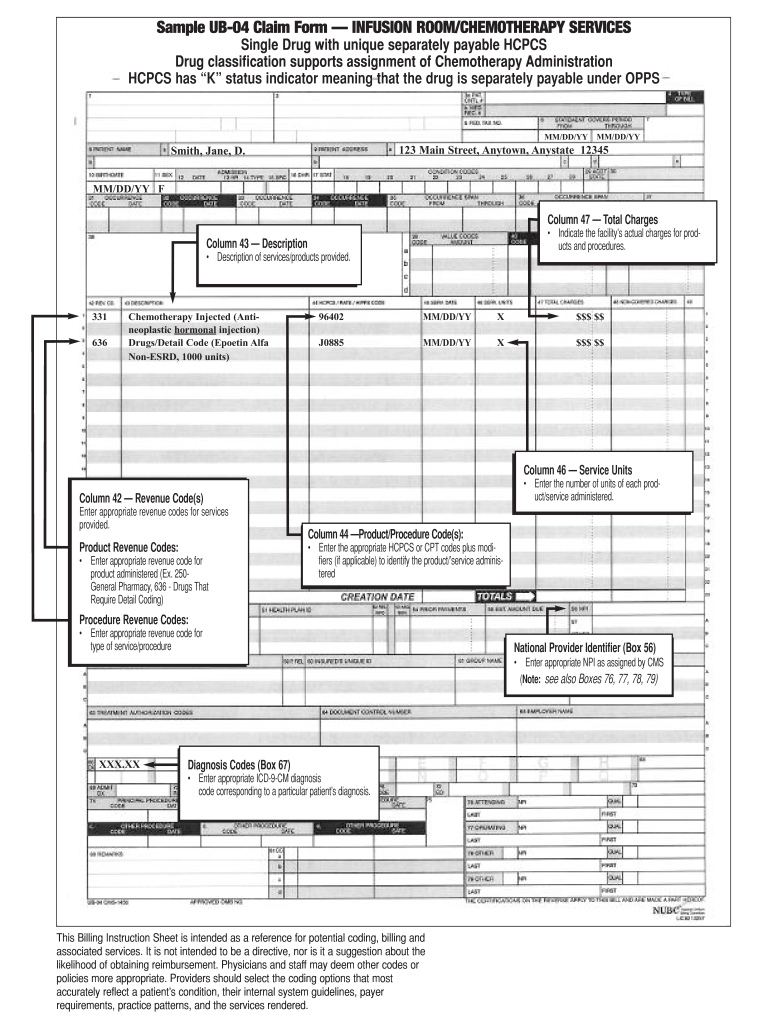
How to get a clia number. Cdc laboratories enhancing lab quality clia certificates print cdc laboratories that perform clinical testing (except clinical trials and basic research) must adhere to clinical.
Clia travel agency membership now, more than ever, clia travel agency membership is an investment in your agency’s success. You can find the certification application at the how to apply for a clia certificate, including international laboratories webpage. Npi phone lookup taxonomy codes lookup the clia lookup by npi or clia npi crosswalk finds npi records based on npi number, clia number or provider name.
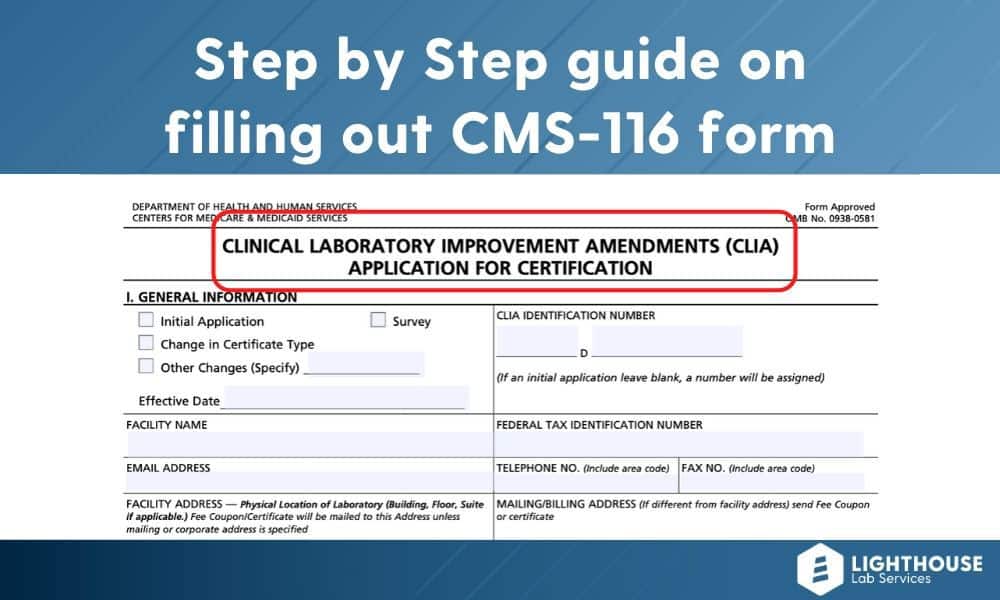
Look up your clia certificate external icon expiration date using your certificate number or laboratory name. This is your personal number to provide clients and vendors to certify you sell travel. Cms clia certification complete general information in section i.
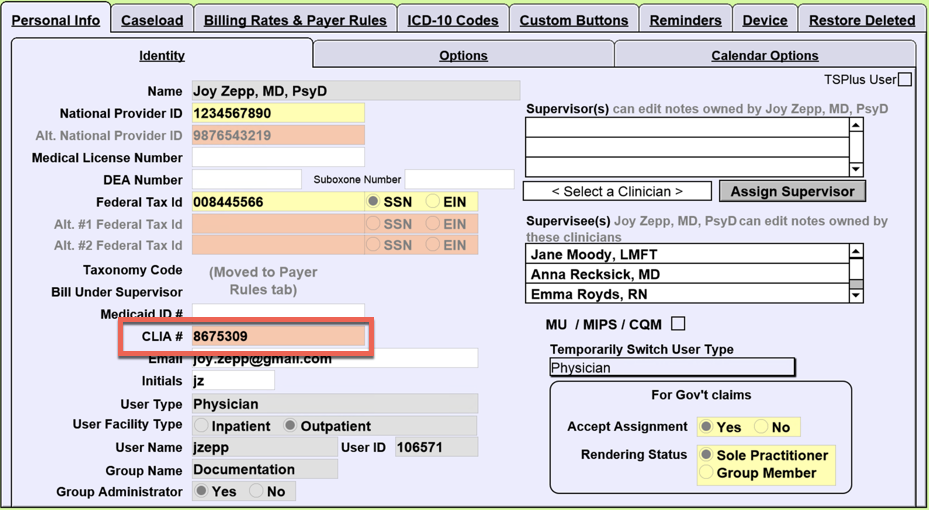
Clia cards contain a unique embarc id number. You should use this number when making. Learn how to report a complaint about a laboratory pdf icon external.
The card also proves your skills to the industry! A clia card is a unique id number that identifies you as a seller of cruises to vendors and suppliers and allows you to book. For waived testing, clia requires that you:
Meet clia certification standards. Will i receive an identifying clia number? Applying from outside the u.s.
The applicant must be at least 18 years of age at the time of application. The applicant must have a remunerated position at the travel agency. It’s called a clia card or number.
This identification number is unique for every submission and is used for tracking the. As part of working with an established host agency like khm travel group, you do not have to obtain your own clia number to obtain the card. The applicant must work at least 20.
To qualify for clia individual agent membership (iam), your affiliated agency must be a current clia travel agency member. This number will be utilized to identify and track your laboratory throughout its entire history. Membership raises your professional credibility.
The clinical laboratory improvement amendments (clia) is the federal. Here, we answer some key questions about clia and clia waived tests.




.jpg?tr=w-1200%2Cfo-auto)